 Institute of Applied and Computational Mathematics
IACM is one of the few research institutes in Europe dedicated to promoting the use of advanced mathematics in natural sciences and engineering
Institute of Applied and Computational Mathematics
IACM is one of the few research institutes in Europe dedicated to promoting the use of advanced mathematics in natural sciences and engineering
Computational Pharmacology
ABOUT
RESEARCH AND DEVELOPMENT ACTIVITIES
Modelling & Simulation in drug development: Modeling and simulation (MS) approaches represent an integral part of drug development, starting from in vitro testing up to all phases of the clinical procedure. The benefits of applying MS methods in drug development include the following: setting the appropriate sampling scheme and estimating the necessary sample size; less human exposure in clinical trials; reducing the cost of drug development; optimizing the clinical study design; allowing for more rapid development procedures; early selection of the most appropriate chemical compounds and/or formulations; gaining more information regarding the properties of the medicines; integrating in vitro and in vivo drug properties; and individualizing dose requirements. Today, the requirements of the European Medicines Agency and the US Food and Drug Administration make the application of MS methods an essential step in drug development.
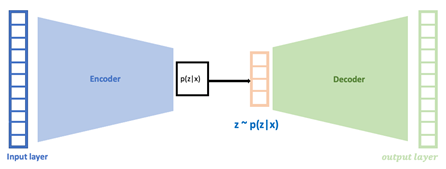
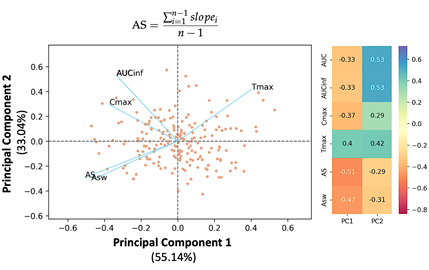
Artificial Intelligence: One of the most rapidly rising activities of the group is to analyze and describe real-world problems in medicine and pharmacy. However, real-world situations can be rather complex and very often cannot be analyzed using typical inferential statistics like t-tests, general linear models (e.g., analysis of variance), the correlation coefficient of Pearson, etc. Thus, additional methodologies are required, such as artificial intelligence, machine learning, multivariate statistics, non-linear mixed effect modeling, non-parametric methods, classification techniques, Monte Carlo methods, signal analysis, and procedures for analyzing time series data. All types of statistical analyses are used in the drug development process and clinical problems.
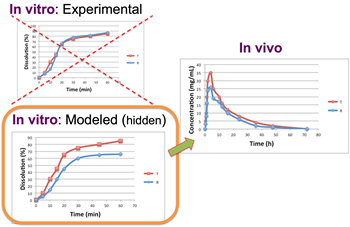
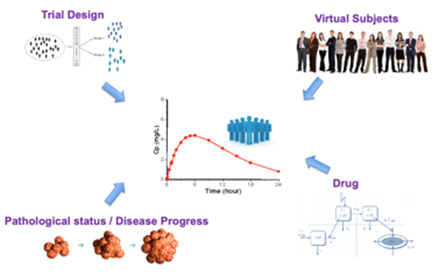
In silico clinical trials: One of the group’s activities is to develop in silico clinical trials (ISCT) in order to assist drug development. ISCT is an expression for clinical trials performed on computers or via computer simulations. ISCT allows predicting the outcome of a clinical trial and testing any condition that could potentially affect the outcome without performing the actual study. Thus, ISCT exhibits many advantages since it allows us to predict the in vivo performance of a medicine, investigate cases not able to be tested in actual practice, evaluate different conditions, optimize study design, adjust the most appropriate dosage regimen, and use a reduced sample size, thus minimizing time and cost during drug development.
Software products: The group works intensively on the development of software and applications used in the R&D departments of pharmaceutical industries. Among others, the group has developed an in vitro-in vivo simulation tool (IVIVS), which is a flexible tool able to predict the in vivo behavior of a formulation and/or the bioequivalence outcome based only on in vitro data. This IVIVS app is useful for selecting the most appropriate formulation during development and the most appropriate clinical trial design and sample size.

Education and Training: The group contributes to the education and training of undergraduate, graduate, and post-graduate students, PhD students, and postdoctoral researchers, as well as pharmaceutical industry scientists, in the areas of modeling and simulation, software development, artificial intelligence and statistics, bioequivalence, population pharmacokinetic modeling, and any computational aspect of the drug development process.
Computational Pharmacology
RESEARCH AND DEVELOPMENT PROGRAMS
COMPLETED PROJECTS
- Development of a Triple Combination Tablet for the treatment of Hypertension 3CT4Hypertension (ΤΕ1ΔΚ 561, ΕΣΠΑ 2014-2020)
PEOPLE
- Vangelis Karalis, Collaborating Researcher
- Georgia Karali, Collaborating Researcher
- Anastasia Tsyplakova, PhD candidate
- Maria Kokkali, PhD candidate
- Anastasios Nikolopoulos, PhD candidate
- Ileana-Maria Theofili, PhD candidate
- Nikodimos Chatzigeorgiou, PhD candidate
CONTACT US
For any information regarding the group please contact:
Computational Pharmacology Group,
Institute of Applied and Computational Mathematics,
Foundation for Research and Technology - Hellas
Nikolaou Plastira 100, Vassilika Vouton,
GR 700 13 Heraklion, Crete
GREECE
Tel: +30 2810 391800
E-mail: This email address is being protected from spambots. You need JavaScript enabled to view it. (Mrs. Maria Papadaki)
Tel.: +30 2810 391805
E-mail: This email address is being protected from spambots. You need JavaScript enabled to view it. (Mrs. Yiota Rigopoulou)